View Press Releases
New Paper from Insilico Medicine Demonstrates Validity of AI Drug Discovery
Artificial intelligence in drug discovery has made serious inroads, with a number of AI-designed drugs now in later-stage clinical trials. But how exactly was AI employed in the process – and where’s the data to demonstrate its validity? Now, for the first time, a new paper in Nature Biotechnology from clinical stage AI-driven biotechnology company Insilico Medicine reveals the entire process behind the company’s lead AI-designed molecule for an AI-discovered target for the chronic, progressive and often fatal rare lung disease idiopathic pulmonary fibrosis (IPF), which impacts 5 million people worldwide.
The drug in question, INS018_055, is one of 31 programs in Insilico’s pipeline developed through generative AI and it is the first AI-discovered and AI-designed drug to reach Phase II trials with patients. Understanding the pathway for this lead drug – and the selected target, TNIK – provides a valuable blueprint to those in the pharmaceutical and biotech industries looking to better understand this game-changing technology. Insilico Medicine is a pioneer in utilizing generative AI for drug discovery and development, and first described the concept of using generative AI for the design of novel molecules in a peer-reviewed journal in 2016. Then, Insilico developed and validated multiple approaches and features for its generative adversarial network (GAN)-based AI platform and integrated those algorithms into the commercially available Pharma.AI platform, which includes generative biology, chemistry, and medicine.
“Nowadays, it seems that we read about the virtues of AI, ML, generative design almost daily. There is a feeling that, perhaps, it is overhyped,” says Stevan Djuric, PhD, adjunct professor in medicinal chemistry at the University of Kansas and former head of the global medicinal chemistry leadership team at AbbVie. “However, in this paper, the Insilico Medicine team convincingly demonstrates the power of their proprietary platform which features target identification and validation, medicinal chemistry design and clinical trial components using the aforementioned tools.”
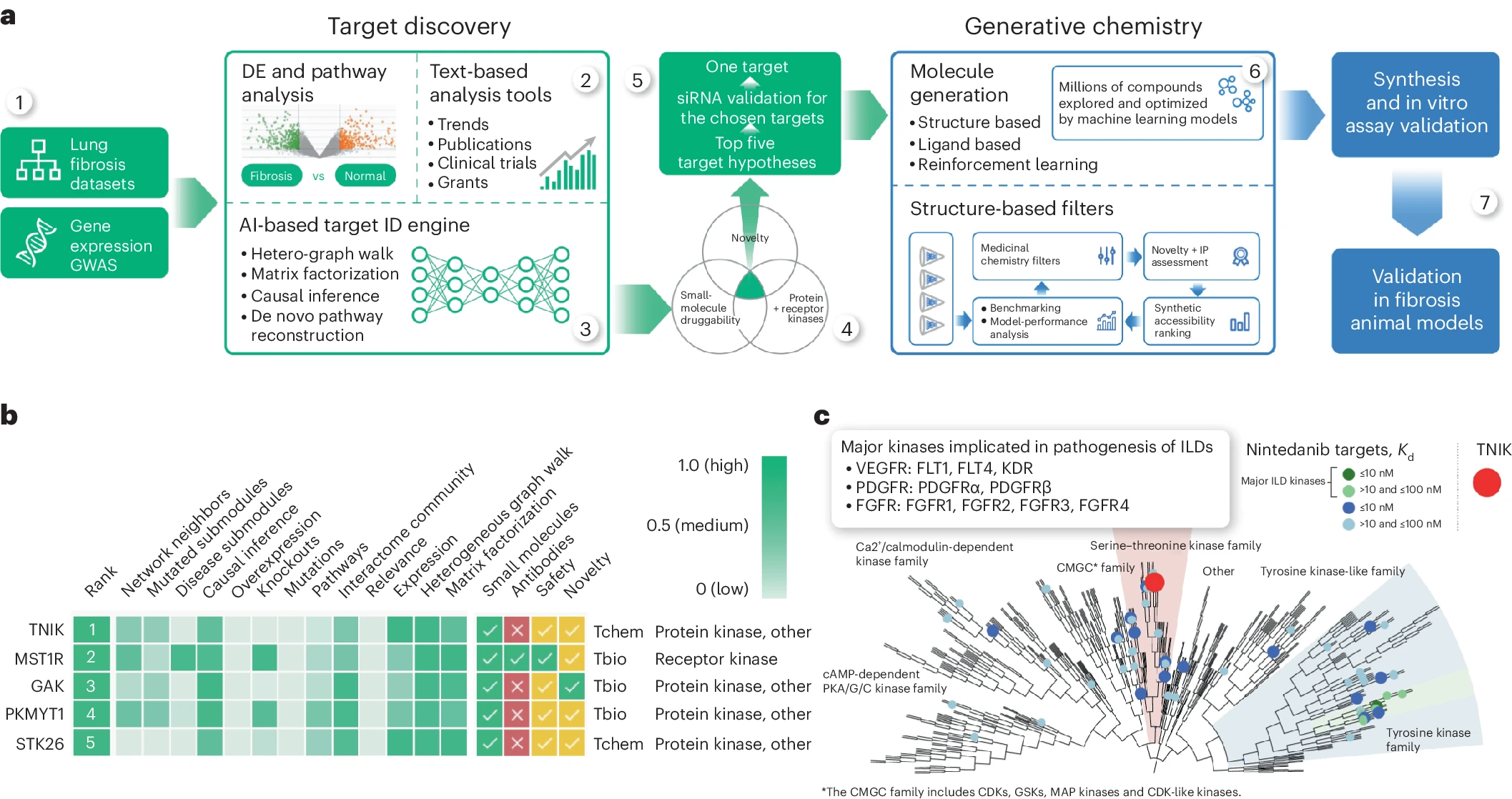
The first hurdle that Insilico’s AI platform, Pharma.AI overcame was to find a potent target implicated in both aging and disease that would make for a promising candidate for its lead program. Insilico focused on fibrosis, a major pathological feature driven by the hallmarks of aging, training its target identification engine, PandaOmics, on omics and clinical datasets related to tissue fibrosis. Although TNIK has never been pursued previously as a target for IPF, PandaOmics identified it as the most promising anti-fibrosis target.
“Although I was skeptical in the beginning, all data that we generated clearly supported Insilico's prediction of anti-fibrotic efficacy,” says Klaus Witte, MD, German Medical Board-certified pharmacologist and toxicologist and preclinical consultant to Insilico. “Looking at the broad set of preclinical and clinical data that is meanwhile available, I'm confident that INS018_055 could become a very valuable treatment option in pulmonary fibrosis and other fibrosis indications.”
With the promising target selected, Insilico scientists used the company’s generative AI drug design engine, Chemistry42, which combines over 40 generative chemistry engines and over 500 pre-trained reward modules, to design a novel small molecule that could be advanced as a new treatment, using the available crystal structures of the TNIK kinase as a starting place. They directed Chemistry42 to make small molecules capable of binding to both TNIK and adjacent allosteric pockets and chose the AI-designed structures that demonstrated the best synthetic accessibility, novelty and medicinal chemistry properties for further testing, ultimately synthesizing and testing less than 80 compounds – much lower than traditional drug discovery.
One promising hit candidate emerged and was further optimized to increase solubility, promote a good ADME safety profile, and mitigate unwanted toxicity while retaining its remarkable affinity for TNIK. This was the lead molecule, INS018_055.
“Insilico is literally AI from A to Z,” says Michael Levitt, PhD, 2013 Nobel Prize winner in chemistry. “They not only identified a novel target, but also accelerated the whole early drug discovery process and they've quite successfully validated their AI methods in the TNIK program.”
INS018_055 has now been advanced through multiple clinical trials and exhibited excellent performance. In November 2021, 9 months after Insilico scientists nominated INS018_055 as a preclinical candidate, the first healthy volunteers were dosed in a first-in-human (FIH) microdose trial of INS018_055 in Australia with results exceeding expectations.
This was followed by Phase I trials carried out in New Zealand and China, where INS018_055 was tested in 78 and 48 healthy subjects, divided into cohorts focusing on a single ascending dose (SAD) study and multiple ascending dose (MAD) study which demonstrated favorable safety, tolerability, and pharmacokinetics (PK) profiles and supported the initiation of the Phase II studies.
Now, INS018_055 is in twoPhase 2a clinical trials for the treatment of IPF in the United States and China – the first AI-designed drug for an AI-discovered target to reach this milestone. The studies are randomized, double-blind, placebo-controlled trials designed to evaluate the safety, tolerability and pharmacokinetics of the lead drug and the trials will assess the preliminary efficacy of INS018_055 on lung function in IPF patients.
“From my perspective, the progress of INS018_055 has significant implications for the drug discovery field,” says Insilico founder and CEO Alex Zhavoronkov, PhD. “It not only serves as a proof-of-concept for Pharma.AI, our end-to-end AI-driven drug discovery platform, but sets a precedent for the potential of generative AI to accelerate drug discovery. Using the publication as a guide, one can extrapolate how generative AI drug discovery tools may streamline early discovery efforts. We anticipate that the expanded application of this platform will address challenges facing industry R&D, including cost and efficiency, and accelerate the delivery of innovative therapies to patients.”


