View Press Releases
Clinical Trials Market Size USD 84.43 Billion by 2030
The size of the global clinical trials market is expected to exceed USD 84.43 billion by 2030 at a compound annual growth rate of over 5.7 percent, from an estimated USD 53.87 billion in 2022, according to a Canada-based organization Precedence Research.
Factors such as growing prevalence of chronic disorders, increasing number of clinical trials in developing regions, growing number of biologics, increasing demand for advanced treatments such as personalized medicines, outbreak of viral diseases, increasing cases of cancer globally, growing geriatric population and growing research and development expenditure are propelling the clinical trials market expansion across the globe.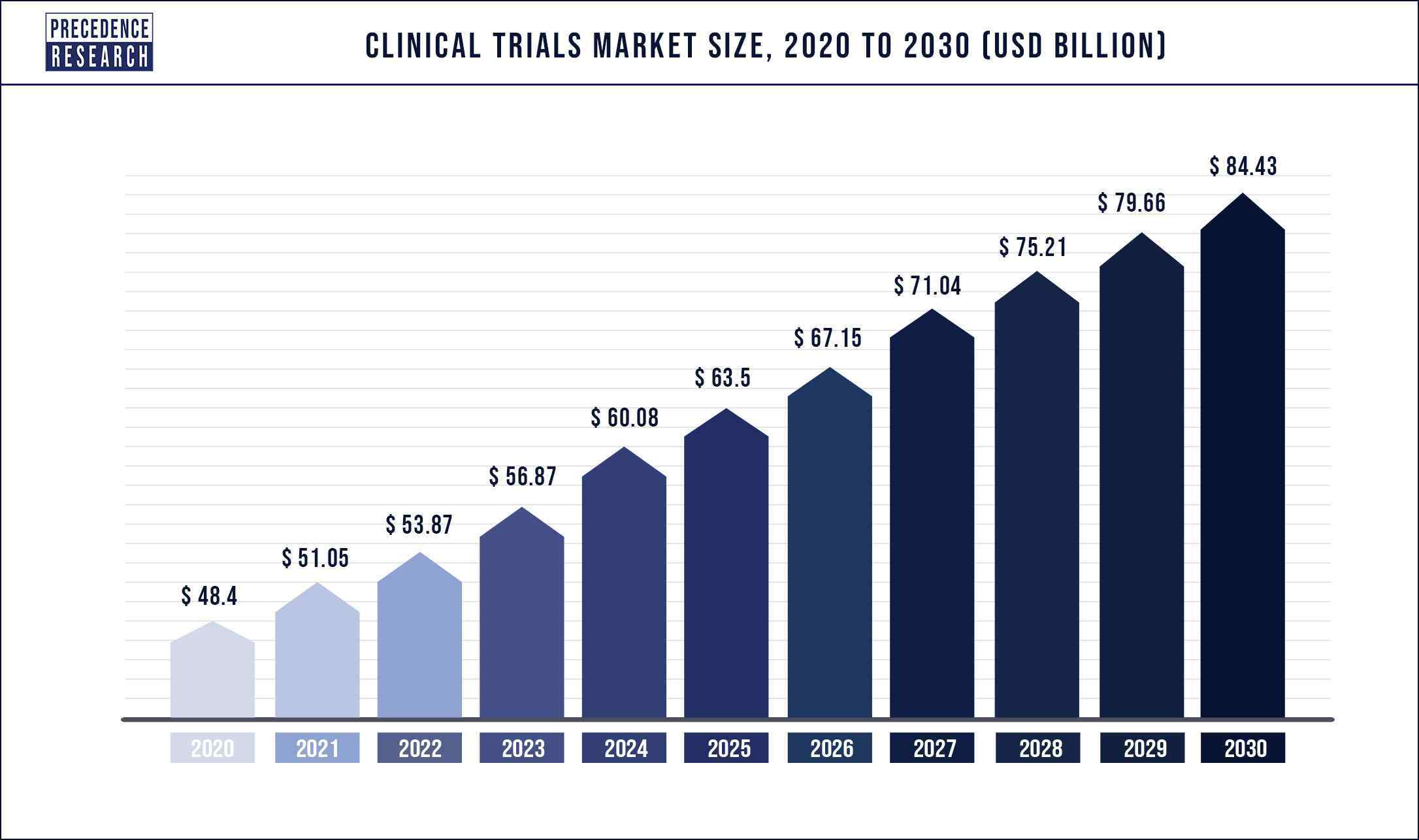
The clinical trials market is expanding because to the rising frequency of chronic disorders and the rising demand for clinical trials in developing nations. The clinical trials market is fueled by the increasing number of biologics on the market and the demand for contract research organizations to undertake clinical studies. In addition, various biotechnology and pharmaceutical companies are now conducting clinical trials for severe chronic and infectious disorders such as HIV and cancer, which will help to expand the clinical trials market.
Regional Snapshot
Asia-Pacific is the largest segment for clinical trials market in terms of region. This is attributed to the growing availability of a broad patient pool, making candidate recruiting easier. The global COVID-19 pandemic is also a major contributor to the clinical trials market expansion.
North America region is the fastest growing region in the clinical trials market. This can be linked to the North America region’s increased research and development adoption of new clinical research technology. Furthermore, favorable government assistance for clinical trials in the U.S. is expected to stimulate demand.
Get a Free Sample Copy @ https://www.precedenceresearch.com/sample/1185
Clinical Trials Market Scope
| Report Highlights | Details |
| Market Size | USD 84.43 Billion by 2030 |
| Growth Rate | CAGR of 5.7% from 2022 to 2030 |
| Largest Market | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Phase, Study Design, Indication |
Report Highlights
- Based on the phase, the phase III segment dominated the global clinical trials market in 2020 with highest market share. The fact that a phase III study covers a high number of individuals and is the most expensive phase of a trial among all segments contributes to the clinical trials market growth.
- Based on the study design, the interventional design segment dominated the global clinical trials market in 2020 with highest market share. Interventional studies account largest percentage of all investigations, with the bulk of them using medicines or biologics, followed by clinical procedure, behavioral, and device intervention studies.
- Based on the indication, the oncology segment dominated the global clinical trials market in 2020 with highest market share. The oncology is the study of tumors. Tumors have the potential to be life-threatening in a large number of individuals, necessitating the development of better and more advanced treatments for diverse forms of tumors.
Market Dynamics
Drivers: Surge in demand for outsourcing clinical trials
The demand for efficient, fast-paced, and trustworthy clinical trials programs is expected to expand as the demand for novel medications and improved medical technologies grows. Furthermore, the medication development process is exceedingly dangerous for biotechnology and pharmaceutical businesses, with much lower approval rates and accompanying expansive expenses. As a result, outsourcing the clinical trials program to several contract research organizations (CRO) has been determined to save the pharmaceutical corporation substantial time and money. Regional penetration of specific contract research organizations (CRO) has also been documented. Thus, the surge in the demand for outsourcing clinical trials is propelling the growth of the clinical trials market growth during the forecast period.
Restraints: High cost of clinical trials
The clinical trials market’s services are costly. In the forecast period, market labor costs are a restricting factor for the market growth. Patenting and contracting for the clinical trials market is a complicated process. As a result, the clinical trials market’s labor costs are high. The cost is an issue since it reduces demand in a few markets. The clinical trials services must be cost-effective in most businesses. The high cost, on the other hand, raises the industry’s overall operating costs. Thus, the high cost of clinical trials is hindering the growth of the clinical trials market during the forecast period.
Opportunities: Growing use of predictive analytics
Several firms are already using predictive analytics techniques such as artificial intelligence and machine learning to construct models and advise choices. Given the wealth of health data now available to clinical trial investigators, predictive analytics tools can be used in clinical trial design to identify patient characteristics that are more likely to respond to a specific treatment pattern, thereby increasing success rates and lowering risk in large, multi-center clinical trials. As a result, the growing use of predictive analysis is creating lucrative opportunities for the market growth during the forecast period.
Challenges: Stringent government regulations
Conducting clinical trials in different nations comes with a slew of regulatory challenges that could stymie market growth. The restrictions can be simplified with the cooperation of multiple regulatory authorities, however there is now a slow operating speed. Several nations also demand local language translation, as well as import and export authorization and the presentation of data on local patients. As a result, a complicated regulatory structure complicated regulatory structure combined with a considerable language barrier could stifle regional development.
Top Players contending in the Market:
The companies focusing on research and development are expected to lead the global clinical trials market. Leading competitors contending in the global clinical trials market are as follows:
- Parexel
- IQVIA
- Charles River Laboratory
- Omnicare
- Kendle
- Chiltern
- Pharmaceutical Product Development, LLC
Major Market Segments Covered:
By Phase
- Phase 1
- Phase 2
- Phase 3
- Phase 4
By Study Design
- Observational
- Interventional
- Expanded Access
By Indication
- Autoimmune/Inflammation
- Rheumatoid arthritis
- Multiple Sclerosis
- Osteoarthritis
- Irritable Bowel Syndrome (IBS)
- Others
- Pain Management
- Chronic Pain
- Acute Pain
- Oncology
- Blood Cancer
- Solid Tumors
- Other
- CNS Condition
- Epilepsy
- Parkinson's Disease (PD)
- Huntington's Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle Regeneration
- Others
- Diabetes
- Obesity
- Cardiovascular
- Others
By End User
- Hospital
- Laboratories
- Clinics
By Application
- Vaccine
- Cell & Gene Therapy
- Small Molecules
- Other Applications
Regional Segment
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Southeast Asia and Rest of APAC)
- Latin America (Brazil and Rest of Latin America)
- Middle East and Africa (GCC, North Africa, South Africa, Rest of MEA)
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Buy this Premium Research Report@ https://www.precedenceresearch.com/checkout/1185
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com


