CB Insights Recognizes Proscia Among Most Promising Digital Health Companies Of 2024
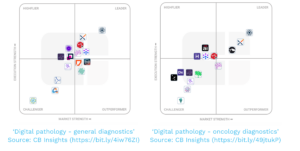
Philadelphia – December 5, 2024 – CB Insights named Proscia®, a global leader in AI-enabled pathology solutions for precision medicine, to its 2024 Digital Health 50 list of the world’s most innovative private digital health companies. Proscia is also ranked as the top vendor in CB Insights’ ‘Digital pathology – general diagnostics’ and ‘Digital pathology – oncology diagnostics’ Execution, Strength, and Positioning (ESP) market matrices.
 CB Insights selected the Digital Health 50 based on analysis of 10,000+ startups from 9 countries using its proprietary metrics — Commercial Maturity and Mosaic scores — along with additional data on partnerships, patents, and leadership. It supplemented this analysis with direct company submissions via Analyst Briefings.
CB Insights selected the Digital Health 50 based on analysis of 10,000+ startups from 9 countries using its proprietary metrics — Commercial Maturity and Mosaic scores — along with additional data on partnerships, patents, and leadership. It supplemented this analysis with direct company submissions via Analyst Briefings.
“The 2024 Digital Health 50 cohort highlights high-momentum companies driving meaningful change across healthcare,” said Amrit Panjabi, Intelligence Analyst at CB Insights. “This year’s winners are at the forefront of bringing an AI-driven infrastructure to healthcare, transforming diagnostics, and bringing condition-specific care platforms to patients and providers. These companies are accelerating the transformation of healthcare delivery, streamlining workflows, and advancing patient outcomes globally.”
The ESP matrix leverages CB Insights’ data and analyst insight to identify and rank leading private companies in a given technology landscape. This proprietary methodology integrates thousands of unique data points to determine a company’s eligibility and positioning relative to its peers. Through distinct stages of analysis, companies are selected for final inclusion in the matrix based on overall quality as well as strength of signals pertaining to their Market and Execution. Each company is evaluated against the same criteria in order to arrive at an objective, visual representation of the market. Proscia was ranked as the leader among 15 companies in both the ‘Digital pathology – general diagnostics’ and ‘Digital pathology – oncology diagnostics’ ESP market matrices.
“We are honored to be recognized as a leader at such a pivotal moment for pathology,” said David West, Proscia’s CEO. “Rapid advancements in AI are unlocking new sources of value for digitization in the precision medicine paradigm at an unprecedented pace. We look forward to helping our customers continue to capitalize and elevate their role in the future of healthcare.”
As digital pathology advances a new wave of precision medicine, Proscia’s Concentriq® software platform, AI, and real-world data are fueling the development and use of the next novel therapies and diagnostics. The company counts 12,000+ pathologists and scientists across both 14 of the top 20 pharmaceutical companies and major diagnostic laboratories in its user base and further amplifies its reach through distribution partnerships with Agilent Technologies and Siemens Healthineers. Among Proscia’s more recent accomplishments, the company secured FDA 510(k) clearance for its Concentriq AP-Dx and released findings from a study on the impact of AI-enabled workflows with Quest Diagnostics, a leading provider of diagnostic information services.
About CB Insights
CB Insights is an AI super analyst for market intelligence. It delivers instant insights that help you bet on the right markets, track competitors, and source the right companies. This AI super analyst is powerful because it is built on the validated database of companies and markets that CB Insights is famous for. To learn more, please visit www.cbinsights.com.
About Proscia
Proscia is a software company accelerating pathology’s transition to a digital, data-driven discipline and enabling AI to advance precision medicine. Its Concentriq enterprise pathology platform, precision medicine AI portfolio, and real-world data fuel the development and use of novel therapies and diagnostics to drive the fight against humanity’s most challenging diseases, like cancer. 14 of the top 20 pharmaceutical companies and a global network of diagnostic laboratories rely on Proscia’s solutions each day. The company has FDA 510(k) clearance and CE-IVDR certification for its diagnostic software. For more information, visit proscia.com, and follow Proscia on LinkedIn and X.


