Tailocins Play Starring Role In Atomic-Level Horror Show
By Deborah Borfitz
May 13, 2021 | Large protein nanomachines produced by bacteria to selectively annihilate other members of their microbial community could one day be weaponized for wiping out undesirable inhabitants of the human microbiome known to cause ill health and disease. At least that is the vision of Vivek Mutalik, a research scientist at Lawrence Berkeley National Laboratory (Berkeley Lab), who is fascinated with the sometimes lethal “tailocins” that resemble phages but for the absence of a head (aka capsid) to confine its genetic material.
How tailocins select targets for attack is not well understood, but the behavior is clearly altruistic since other cells of the host lineage are spared, says Mutalik. Tailocins are only deadly to specific strains of bacteria—presumably, their rivals—earning them the nickname "homing missiles," as impressively illustrated in a painting by his 15-year-old daughter Antara for the uneducated masses.
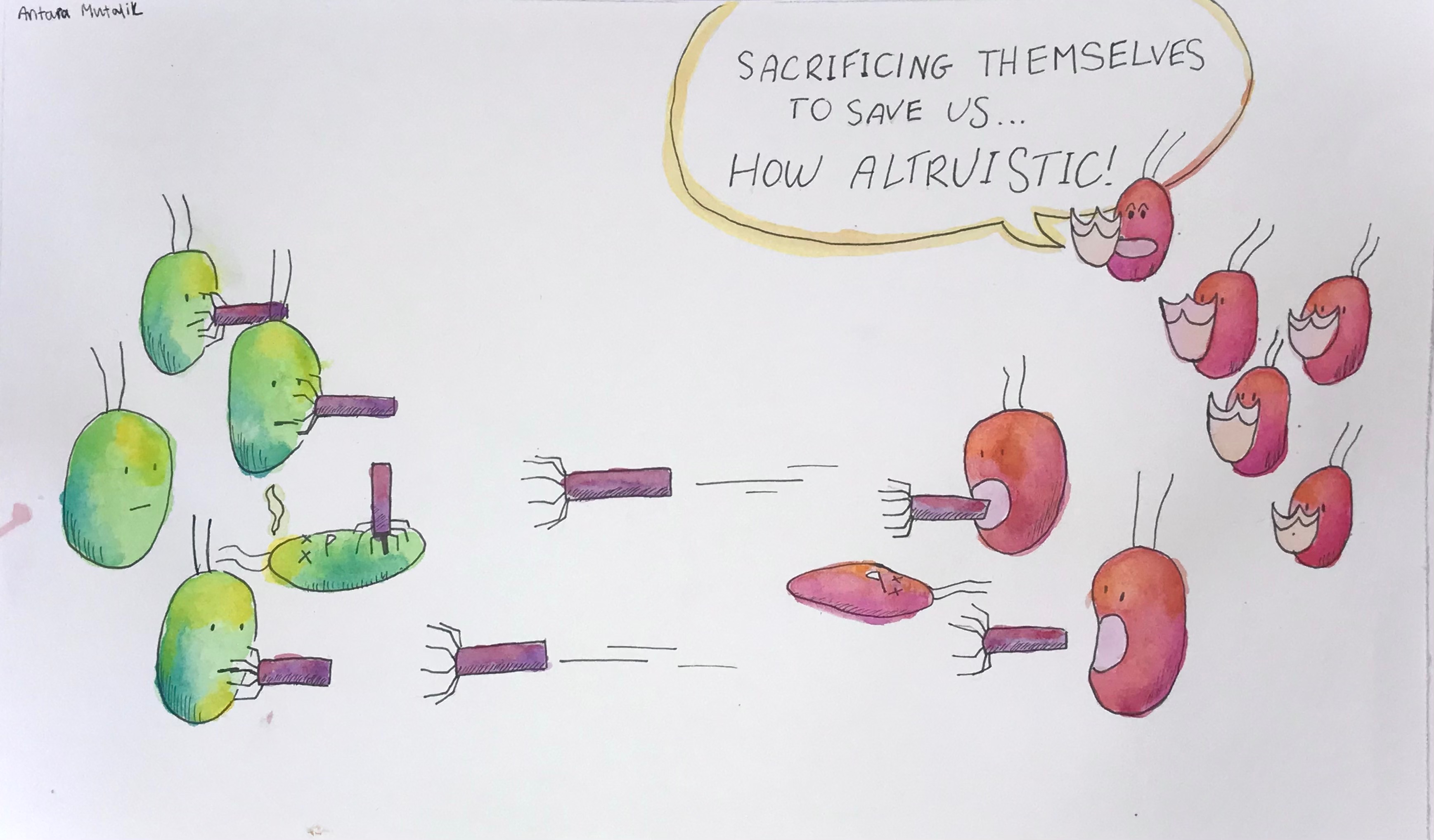
The needle-shaped particles are also conveniently resistant to the tailocins they produce, much like small molecule antibiotics are resistant to self-intoxication. But the bacteria from which they are derived die because the tailocins make a dramatic exit through the membrane of the producing cell.
Tailocins seem to be made in response to stressful conditions, Mutalik says. As predators, they kill their bacterial prey by stabbing a hole in the membrane of cells with their spring-powered needle plunger. With no time to respond, the cells lose their contents and collapse.
It is quite the horror show. In fact, scientists plan to film a “microscopic slasher movie” using the advanced imaging facilities at the Berkeley Lab to captures all the gory, atomic-level details, he says.
Because tailocins kill closely related strains of bacteria, they have been proposed as precision antibacterial agents for therapeutic applications, says Mutalik. But they remain a relatively uncommon topic of scientific study.
Not Phage
Tailocins, discovered in the 1950s, have interchangeably been referred to as “phage tail-like bacteriocins” or “pyocins,” which are technically tailocins specific to the Pseudomonas species, says Mutalik. The favored name in his research circle is “headless zombie” phages or viruses that parasitize bacteria.
By whatever name they might be called, the phage-like particles can arise from a single strain of bacteria that produces all the required genetic information—and it is clustered in the same region of the bacterial genome.
Phages and tailocins share an evolutionary and morphological relationship, but their predation tactics differ significantly, Mutalik points out. Phages will sometimes hijack the cellular machinery of bacteria, making copies of themselves and then busting out to go after other bacteria—much like the modus operandi of the SARS-CoV-2 virus infecting human cells. Other times phages will insert themselves into the bacterial genome and burst open to begin the replication process in response to a stress trigger, such as DNA damage.
Tailocins contain no genetic material of any kind. That is, there is no RNA or DNA to release through the holes they bore.
Bacteria also mount a counterattack when phage strike, perhaps by activating phage-targeting enzymes such as CRISPR-Cas systems or by making it difficult for phages to reproduce once inside the cell, continues Mutalik. No such responses are seen when tailocins are the aggressors.
Tailocin Sensitivity
In a study recently published in The ISME Journal (DOI: 10.1038/s41396-021-00921-1), Mutalik and his colleagues from UC Berkeley and Berkeley Lab comprehensively described the genetic factors behind sensitivity to tailocins in pseudomonads isolated from groundwater at a site (Oak Ridge Field Research Center in Tennessee) contaminated with high nitrate and heavy metals by nuclear programs over the last century. Previously, little was known about tailocin sensitivity and the mechanisms behind resistance to self-intoxication.
Earlier research demonstrated that tailocins use receptor-binding proteins on the tip of their tails to recognize different sugar and fat molecules on the membrane of their bacterial targets. It has also been shown that changing those proteins can cause tailocins to attack different bacteria.
Key findings with the new study are that most of the 12 Pseudomonas strains in the collection encode and produce tailocins that have a highly specific pattern of lethality. Tailocin sensitivity is tied to a particular surface polysaccharide (O antigen) as well as lipopolysaccharide thinning. The discoveries can serve as a foundation for future studies on the utility of tailocins for manipulating microbiomes.
Tailocins are separately being studied by researchers at the Berkeley Lab in their work with the multi-disciplinary, multi-institutional Ecosystems and Networks Integrated with Genes and Molecular Assemblies (ENIGMA) program funded by the U.S. Department of Energy, Mutalik shares. Specifically, they are investigating antagonistic interactions between a few Pseudomonas strains found at the Oak Ridge Field Research Center that might be using tailocins to fight for survival.
Potential Applications
Understanding the targeting mechanisms of tailocins might enable them to be deployed for any number of applications, including ecological processes like carbon sequestration and nitrogen processing, Mutalik says. In medicine, tailocins might also have important therapeutic applications in eliminating bacteria from human microbial communities once scientists have identified the bad actors.
Bacteria could easily be coaxed into producing tailocins in the lab for this purpose, says Mutalik, by treating bacterial cultures with DNA-damaging agents such as mitomycin C, hydrogen peroxide, or UV light. Likewise, it would be simple task to insert the genes into culturable strains for mass production.
Pylum Biosciences holds much of the intellectual property in this arena and has developed a new class of narrow-spectrum, bacteria-killing agents designed to address the rampant spread of drug resistance and unintended damage to healthy protective bacteria associated with the use of broad-spectrum antibiotics. Clinical studies have yet to be done.
Mutalik, meanwhile, has been working with colleagues at the Berkeley Lab to develop genetic tools for non-model organisms to better understand how inhabitants of microbial communities interact. Many mysteries remain. For example, how exactly is bacteria being killed? And can tailocins be designed to deliver a protein or therapy to target pathogens detected somewhere else, potentially the other side of the world?
Real-world applications, for any purpose, are probably at least two or three years away, he says.


